-
Posts
4441 -
Joined
-
Last visited
Content Type
Profiles
Forums
Store
Downloads
Recruiting - 2020
2019-2020 Football Season
Football
Entertainment
Sports
News and Business
Cloak Room
Transfer Portal
Recruiting
Events
Posts posted by triplehorn
-
-
25 minutes ago, Judge Roybeanbag said:
Watching that golf clip, looks like he’s losing his balance and staggering around a couple times.
It's hard to tell, but it looks like he might be knock-kneed. You can see it occasionally with the right camera angle when he's walking.
Spoiler
It might explain why he wears pants tailored for MC Hammer - to hide his embarrassment over the uncoordinated looking contour of his legs. When you get a huge gut and ass sitting on top of wobbly alignment, you might resemble a walking game of Jinga.
-
33 minutes ago, TwiceHorn said:
I read the article and they "retracted" the equivalent statement.
Special Ops has their share of meatheads and psychos, it's still an insult to that community.
Why on earth would BorTac be deployed in Iraq or Afghanistan?
32 minutes ago, Nivek said:
Where is the evidence that antifa loaded a gun and shot this buffoon? I am slightly more likely to believe this individual was killed by one of his Rambo-wannabe friends.To me these two quotes might have something in common. While there are extreme left wing folks out in the streets, a violent "Antifa" movement is far more myth than actual men on the ground. There is no meaningful history of domestic terrorism and murder. There is legitimate concern that non-left agent provocateurs amongst peaceful protesters are inciting violence to create an opportunity. That doesn't necessarily mean they are fringe RW gun nuts, right? So BorTac is on it. What purpose could it serve to have a BorTac presence there? Is BorTac loosely more likely to draw in white supremacists than other traditional federal LE bodies? Is it less discliplined than our military command structure? If you need some loyal operatives temporarily folded into a scene to act in a provocative way inconsistent with best conduct and standard rules, or worse, having a non-traditional less recognizable outside team inserted to pull it off would be one way. It's pretty clear this is in Erik Prince's orbit. He's employed a few mercs over the years. To be clear, I have no evidence of agent provocateurs, but it's fishy as hell and with Prince and Blackwater, we're talking about people with a history of slaughtering civilians. BTW, one of Prince's current initiatives with Frontier Services Group is building internment concentration camps for the Chinese mass detention of the Uighurs. Very fine people.
-
2 minutes ago, Brandywine said:
He should be removed just for retweeting. A President is supposed to be above all of this childish attitude. Anyone with the nuclear codes should act more mature or else be kicked out.
I can tell you that outside of a few square blocks in downtown Portland where a couple Federal buildings are, you wouldn't know it's happening. There is no sense of panic or it being unsafe outside of that concentrated theatre. Listening to Trump brazenly embellish the larger situation to sow fear and division, it only underscores that this is unfolding along some kind of script. Right now, Blackwater (Erik Prince) worldwide website is selling a commemorative tee-shirt 'summer of love' depicting Portland skyline going up in flames. It's not about acting mature when dark arts are in play. Trump is a willing clear and present danger to our own stability.
-
 1
1
-
 1
1
-
-
PNW Charlottesville reprise
-
yeah, that'd be a positive ID.
other pics in there
Spoiler

From the twitters spoilered above, it's pretty clear there are extreme right and left, both tracking each other and instigating.
-
Looks like he's strapped with 3 holsters, right hip has a piece in it. Riot shield on his chest. gloves may or may not be fitted with reinforced knuckles.
-
-
3 minutes ago, Biff Tannen said:
It's quite clear that, at best, the president of the United States is on drugs consistently, right? I mean, that's 25th amendment shit right there. What a time to be alive.
More than golf, it's probably the main reason he spends so much time at his properties. hookers and blow. He can't let it all hang out at the WH.
-
4 minutes ago, Jive Turkey said:

-
 3
3
-
 1
1
-
 1
1
-
-
Evidently the person shot and killed downtown last night was a right wing militia type. Sounds like he took a shot, missed, and caught return fire. Safe to say it's not just RW fringe that are carrying.
-
zero post-convention bounce
-
Erik Prince finger prints on this
This is a tee is being advertised on the blackwater worldwide website right now, saying it'll be gone by this Tuesday.


-
Belongs in the right wing violence thread, but this is what's happening in PDX now:
-
 1
1
-
-
2 hours ago, Bevo said:
Fuuuuck. Disengage. The next time I see someone mention HCQ, I'm passing on their address to Junior Miller and TexasTow.
1 hour ago, Homercles said:Yeah this thread has devolved from generalized covid medical advice to Triples Everlasting Advocacy for Borderline Adequate Group Studies...Operation TEA BAGS.
eh, 'Murica fuck yeah! There is no medical discussion. /thats the joke.
Today, it's good to see that individual states are coming around in real time and letting docs and patients consult and decide. I just now realized Oregon lifted its outpatient use restriction in July. Texas' outpatient use restriction ended in July as well. Resource for actively updated state regulations and medical contact information.
-
 1
1
-
-
In 2016, Congress passed the 21st Century Cures Act which formalized the FDA's use of Real World Evidence (RWE) to support regulatory decision making, including approval of new indications for approved drugs. RWE includes an expanding landscape of non-RCT derived data, including use of observational studies. I'm sure some posters here are way more familiar with FDA integration of RWE than me, and perhaps they can add meaningful insight.
I reference this act of Congress to show that the FDA's use of observational studies making drug approval decisions is an actual initiative. It's increasingly accepted that RCT orthodoxy has real world limitations in optimizing health care decisions (particularly during a novel pandemic). There does not appear to be a well defined set of limits for what clears the bar for adding a new indication for use of an already approved drug. The meta-analysis of FDA drug and device approvals I linked appears to only apply to a subset of new drugs and devices that have no prior approval. FDA approval in their study showed a higher bar for non-RCT data which is entirely understandable in order to account for potential bias and covariates in non-RCT studies.
So what is the pattern of FDA granting approval for a new indication of an already approved drug using non-RCT data? The FDA does this. An act of Congress sanctions it. I'd expect that the threshold for a new indication for an already approved drug is lower than that for a new drug with no prior approval involving basic safety with use. In the meta I linked, the new drugs and devices they examined gained FDA approval using non-RCT data at a 1 in 10 clip. FDA approval is happening when a treatment effect with a positive Relative Risk of 5 or 10 is demonstrated. How generalizable is this rate of FDA approval based off of observational studies across the range of applications, not just for drugs with no prior approval, but for approved drugs seeking approval for a new indication?
In this covid pandemic, I'm also interested to better understand how RWE and non-RCT data overlaps with FDA granting a new emergency use authorization, especially with already approved drugs. Realize remdesivir got EUA based off a single study that showed no mortality benefit, only a 4 day shorter hospital stay. Today's subject is weak evidence in support of pushing ahead with convalescent plasma. In contrast, today we have dozens of studies, many but not all of which are observational, providing consistent and overwhelming evidence that early use of FDA approved HCQ can lower risk of death by 30-40% in Covid- when there are no early alternatives.
Right now more than anything, this leads to it being hard to see that much of anything matters as it relates to prior standards. When you take a step back, what is happening now in the US does not compute. Across a variety of examples related to policy implementation, testing, data reporting and transparency, drug approvals, and so on, the CDC and FDA have been co-opted and are not functioning the way we have come to know and trust.
-
2 minutes ago, Brisketexan said:
It shouldn't fail you. It's readily apparent when you look at the mouthpieces for it. It's entirely political. I will do all I can to avoid going CR here, but the interest is in there being a magic bullet treatment that renders COVID a non-issue, so that 1) we can blame paranoid science-types for ruining the economy for no good reason (blame someone else for the fallout), and 2) go back to normal immediately, so the folks in power can get credit for a recovery right now.
The problem is, wanting the magic bullet to be true doesn't protect you from 3) reality, which is that if we go forward with the "COVID is cured, all is well" path, the shit will really hit the fan.
I want there to be a fucking cure, I want there to be a vaccine - my family has a strong vested interest in this being the case. All of my desire won't change the reality that there ain't one of either ready to go. If real, proper studies end up showing HCQ to be a real-deal effective treatment, I'll be fucking thrilled. But there haven't been any yet. Yet there are folks who are selling it as a solution with everything they've got. See the correlation above. There's literally fucking nothing we won't politicize anymore.
You present the choices as mutually exclusive. They're not. You can have treatment interventions to reduce morbidity and mortality while maintaining masking, distancing, and phased re-opening to further and more rapidly extinguish the virus.
When you say "if proper studies end up showing HCQ to be a real-deal effective treatment, I'll be fucking thrilled. But there haven't been any yet." That carries about as much truth, right now, today, as saying HCQ used appropriately under medical guidance in covid is unsafe.
-
44 minutes ago, JohnLocke said:
Ok, I'll bite. There is not RCT results available that A) use hydroxychloriquine with zinc and azithromycin and B) are given early as an outpatient and C) have a large enough sample size of high risk patients to yield statistically significant results.
I tend to be influenced when I see dishonesty and what I have seen is HCQ being discarded as a treatment based on the fact A) it doesn't work well on very sick patients and B) statistically insignificant results, although positive, and needing larger sample sizes, are reported in the mainstream media as if they were negative results.
The 2nd layer of dishonesty has been the attack on the safety of HCQ, a widely used and well understood drug that is OTC in much of the world. In Harvey Risch's words: The combination of hydroxychloroquine and azithromycin has been used for decades in hundreds of thousands of people with rheumatoid arthritis. There is a concern that these medications do change the heart pacing a little and could cause cardiac arrhythmias. However, these arrhythmias are still very rare in people using these medications. People who already have heart arrhythmias or are predisposed to them or have family histories of them should discuss this with their health care providers and see if using hydroxychloroquine plus doxycycline or some other medications would be a better choice.
There is almost no downside to trying this drug if you are at high risk. In Dr. Risch's words again: Hydroxychloroquine alone is not the whole story. It needs to be combined with azithromycin or doxycycline and probably with zinc to make it most effective. The game changer is to aggressively treat people as soon as possible, before they are hospitalized, to keep them from becoming hospitalized in the first place. Hydroxychloroquine plus the other medications is what we know about now. In a few months we may have data on other medications that also work. We just have to start with something now.
The medical profession has been slow to act on data that is not based on RCT's. Shamefully so, when the data can be conclusive in and of itself. I point again to the rather shameful behavior of the medical profession in adopting antibiotics to treat ulcers as exhibit A.
When you are in the middle of a pandemic, you don't have time for RCT results. However, there is a huge mass of data available to sift through that is significant. I give the CDC an F for being prepared and able to do the heavy lifting in that regard.
We'll have much better data in six months. And hopefully, better treatment options to go along with a vaccine.
Keep in mind that AZ was not used with HCQ in the most recent raft of positive studies showing large benefit. Current understanding is that the beneficial mechanism of HCQ in Covid is anti-inflammatory, with the greatest effect being a dampening effect on covid driven maladaptive inflammation. It follows that when you can inhibit it early, you help prevent the process from gaining steam which can lead to a total collapse of medical stability. FWIW, the common pathway they share that I've seen relates to massive concentration of both AZ and HCQ into intracellular lysosomes due to very high volumes of distribution and acid/base properties of their respective amide groups. Lysosomal activation of inflammasomes (increased IL-18 and IL-1B) is a key pathway driving the highly inflammatory process of pyroptosis. Elevated IL-18 is the immuno-regulatory cytokine most associated with mortality in Covid per Yale Med.
Edit: Importantly, keep in mind that the maladaptive immune response this virus drives doesn't just flip on like a light switch in 10-20% of the population. The studies showing abnormalities in cardiac MRI in a high percentage of patients with outpatient uncomplicated illness, and abnormal brain scans in non-hospitalized patients tells us that potential inflammatory damage begins right away in most everyone. It's a race for how fast you can clear the virus before the maladaptive immune inflammatory response overwhelms you. Various factors and pre-existing conditions make one more susceptible to variable levels of inflammation or have an inability to reduce viral load.
-
Just now, Anastasis said:
The referenced subset being discussed, which you brought up, is by definition a set aside group where different thresholds of evidence are used for compassionate use situations. You make it really hard to take you serious.
If you want to stop being a clown, I am happy to read your critical review of the methods and SAP of the last observational study you posted.
Ah, the formidable double-down dodge. What difference, if there is one, exists between the threshold RR for this subset and other drugs and devices outside of it needed for FDA approval based off of non-RCT observational studies? Or are you suggesting that other doesn't exist? That would be something.
-
1 hour ago, Anastasis said:
Are you asking me what is my threshold for non-RCT data when RCT results are available and informative to the question at hand? Stop being a clown triple.
Nope, and you know it. The FDA gives approval for drugs and medical devices based off of non-RCT observational studies, albeit with a higher threshold for efficacy than RCT-based applications. This is known. You dismissed it pointing out that it related to a subset of applications. I'm asking if you have evidence that there is a different (higher) efficacy threshold for drugs and devices for approval outside of the referenced subset. You've ignored that so far, and your default to name calling is a huge tell. You may be right, but until you can back it up with anything, you're running another bluff. And again this is going above and beyond consideration of a drug that already has FDA safety approval and is widely applied for off-label use.
And to your the point of your latest diversion, what RCT trial shows that early stage initiation of HCQ carries no benefit ? There are real world reasons observational studies are employed. When they are replicated 6 times using 35,000 subjects with a relative risk reduction of DEATH by 20-50%, and FDA safety approval has remained intact for over 50 years, it carries a powerful meaning in a pandemic where we're still at 40,000 new cases and >1000 deaths per day with no other early outpatient alternative.
-
Just now, Anastasis said:
They will use observational data when RCT data is not available. And typically in those cases will not rely on observational data exclusively, but will assess within the full context of the available information. And if they are looking at your observational study, they will get elbow deep up in your ass on your methods and statistical analysis. Which is why I asked you to critically review those aspects of the last pre-print you posted.
Still waiting on you to present your non-RCT FDA approval threshold data in your context.
-
31 minutes ago, Anastasis said:
Triple doesn't read nor comprehend what he cites here. That is a subset analysis of breakthrough treatment applications for the period from 2012-2018. It is not reflective of FDA approvals generally. It reflects a small subset of approvals, tilted towards devices and with the drug approvals represented by mostly cancer drugs. There are good reasons why comparison groups may be unavailable, or randomization untenable in those settings. The current situation is totally dissimilar. This is the kind of out right misrepresentation of data that he traffics in constantly and is frustrating.
You're being presumptuous. I presented it not to draw a granular comparison. I presented it not to make a case for FDA approval. The simple point is that regulatory bodies like the FDA and EMA grant use approval using non-RCT data when the effect size is large - larger than thresholds needed for RCT based data - in order to offset effects of bias and covariates in observational studies. The comparison may be a mismatch wrt FDA approvals, but you have not shown that with other relevant data. To the extent there is a mismatch, the FDA threshold RR difference in this example, 5 or 10 vs. 30-40, is large.
What is the FDA approval threshold for non-RCT positive RR findings in your context?
-
9 hours ago, Scheiss Meister said:
I learned a long time ago that if you try to correlate an effect to only one variable when there are many other variables present, and you do not account for those variables, whatever you find will be trash. These studies looking at HCQ but not adjusting for all of the myriad variables inherent in the human population cannot come to valid conclusions.
Keeping it understandable for me and anyone else, this is a useful study that addresses your concerns:
helpful descriptive part of intro:
QuoteA well-designed randomized clinical trial (RCT) is widely considered the most reliable approach for evaluating the efficacy of clinical interventions or assessing the probable benefit of a medical device. However, RCTs often face practical limitations, such as difficulties in accruing patients, or other logistical or feasibility issues. As a result, alternative methods for the evaluation of treatments are increasingly considered, such as the assessment of a health interventions’ effects in nonrandomized, observational studies.1 Although such evaluations often provide results similar to those seen in RCTs,2,3 exceptions are not uncommon.4 The main concern about non-RCTs is that it is currently challenging to predict with confidence when the results of RCTs will be concordant with those of observational studies. The consequences of such a discordance can sometimes be disastrous.5,6
It is essential to explore when observational studies are considered sufficiently credible. Regulatory agencies, such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have accepted that large treatment effects can sometimes obviate the need for RCTs. They have accepted that non-RCTs can be considered reliable sources of evidence, ie, produce results with a low risk of bias, when they generate sufficiently large or dramatic effects that are thought to rule out the combined effects of bias and random errors.7 Therefore, an approach for evaluating when findings in observational studies can be considered credible because of sufficiently dramatic treatment effects is to analyze the approval decisions of the drug licensing authorities. Based on theoretical and simulation studies, several definitions have been proposed to categorize dramatic effects as those treatments displaying relative risks (RRs) of 5 or greater8 or 10 or greater9 or odds ratios (ORs) of 12 or greater.10
Using these definitions, we showed that the EMA approved 7% of treatments based on non-RCTs; between 2% and 4% of these approvals displayed dramatic effects.10 Like the EMA Priority Medicines and Adaptive Pathways programs, the FDA has a program called the Breakthrough Therapy designation (BTD), designed to support the approval of drugs demonstrating substantial (ie, dramatic) improvement over existing therapies and which may not require further testing in RCTs.7
results of this meta:
QuoteAmong 677 drug and medical device applications, 68 (10.0%) were approved by the FDA based on non-RCTs. Estimates of effects were larger when no further RCTs were required (mean natural logarithm of the odds ratios, 2.18 vs 1.12; odds ratios, 8.85 vs 3.06; P = .03). [...]
Overall, 9 of 677 total applications (1.3%) that were approved on the basis of non-RCTs had relative risks of 10 or greater and 12 (1.7%) had relative risks of 5 or greater. No clear threshold above which the FDA approved interventions based on the magnitude of estimated effect alone was detected.
So about 1 of every 10 drug and med device FDA approvals occur based off of non-RCT data. The size of treatment effect plays a critical role when bodies like the FDA and EMA are evaluating a treatment for formal use approval with non-RCT data. The greater the size of treatment effect, the smaller the extent to which effects of covariates and bias can be attributable as the cause of a positive outcome. In this study "dramatic effects" were defined as relative risks (RRs) of 5 or greater or 10 or greater, or odds ratios (ORs) of 12 or greater.
With a "dramatic effects" threshold for FDA approval using a Relative Risk of 5 or 10, look back at this table:

These RR values are big - even for treatments that gain FDA use approval. 6 studies across 5 nations, involving a combined 34,619 subjects, all with highly consistent findings in direction and magnitude which further support a benefit not due to bias or covariates. Keep in mind we're not talking about getting FDA approval for HCQ use to treat Covid in a crowded field of therapeutics. We're desperately looking for anything that could offer benefit when there is no alternative. And at first glance, this body of large studies goes way beyond positive thresholds in non-RCT studies used by FDA to freaking grant approval. FDA approval for safety with HCQ has existed for over 50 years.
QuoteDesigned and controlled studies can and have shown valid evidence that HCQ is not effective enough to outweigh the risks that come with it.
Careful with your imprecise language. Are you referring to early stage HCQ application, or initiating HCQ in severely ill hospitalized patients with glass lungs? Show me the studies, RCT or not, where early stage application had no benefit. That's the main error that keeps looping in peoples' minds. Even Fauci is guilty of this imprecise language when drawing conclusions. RCT's that show no benefit of HCQ initiated in hospitalized patients with advanced illness are useful - no benefit of HCQ if started too late. Critically, that ignores the growing number of large studies finding dramatic positive effects appearing when HCQ is started very early in disease course. There are myriad examples in medicine where timing of the intervention entirely determines outcome effects.
-
tasking now? that's plain dodge. it's what you do when you've got nothing. all too familiar.
-
you and sheister.

.thumb.jpg.9c0199775c3ff812faad98add86df0fb.jpg)

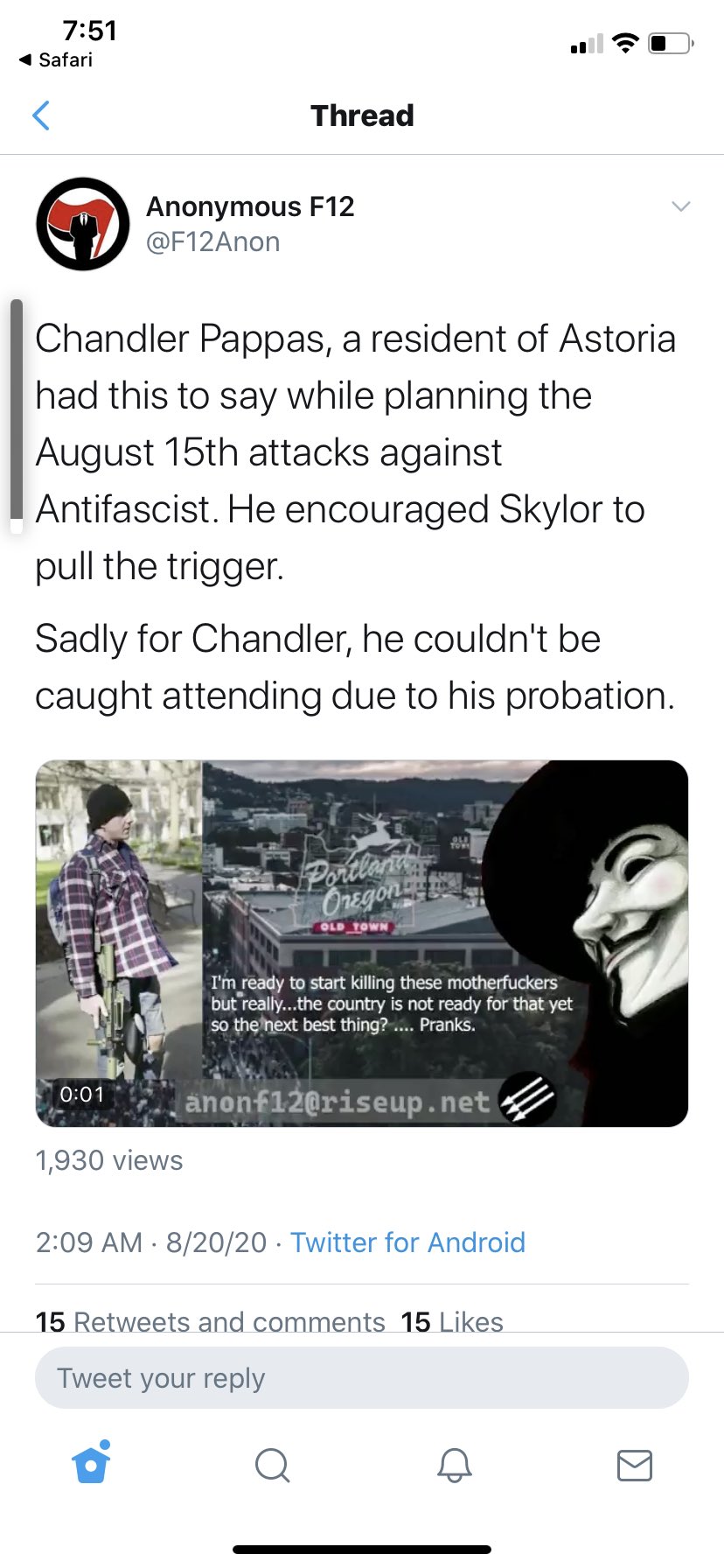
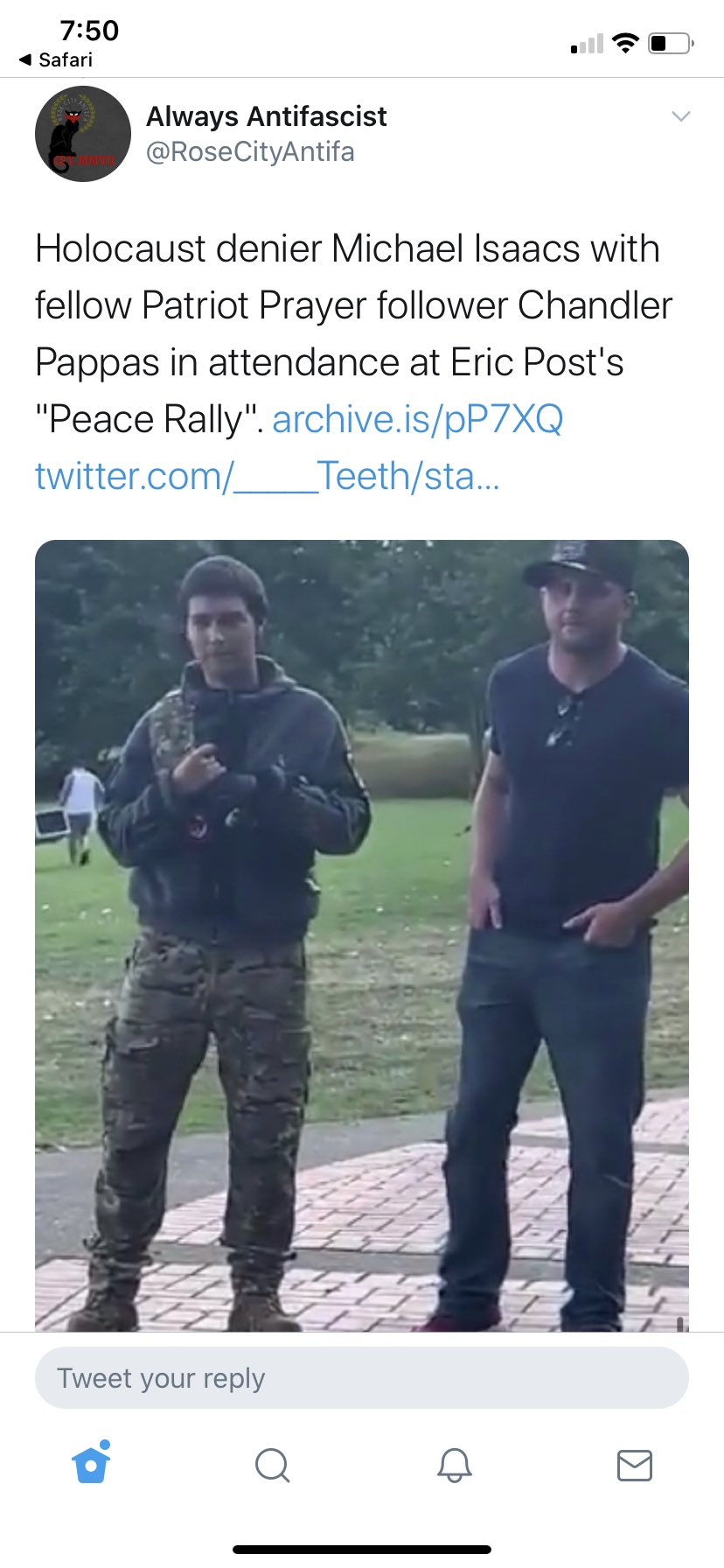





Trump’s Gestapo in Portland
in Cloak Room
Posted · Edited by triplehorn
dp